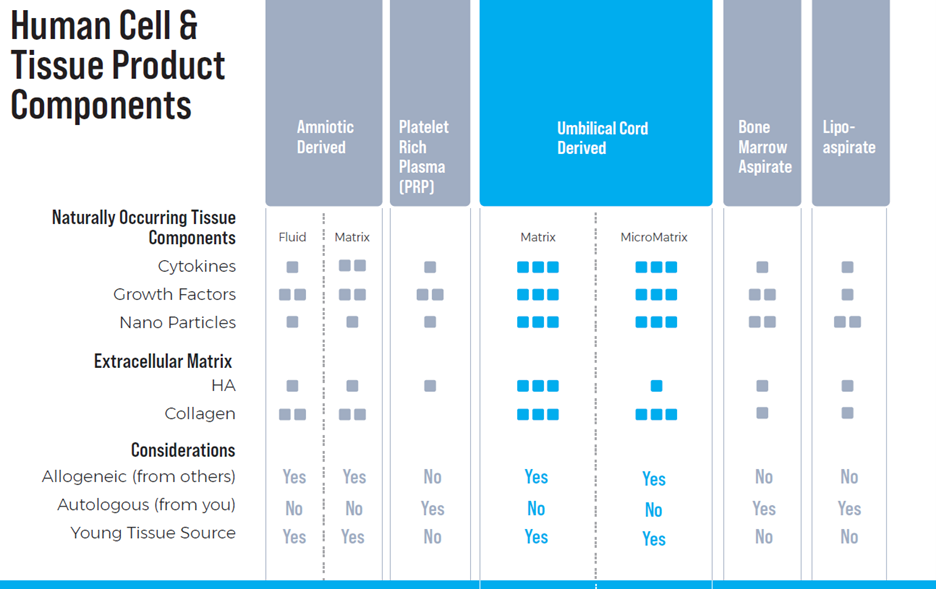
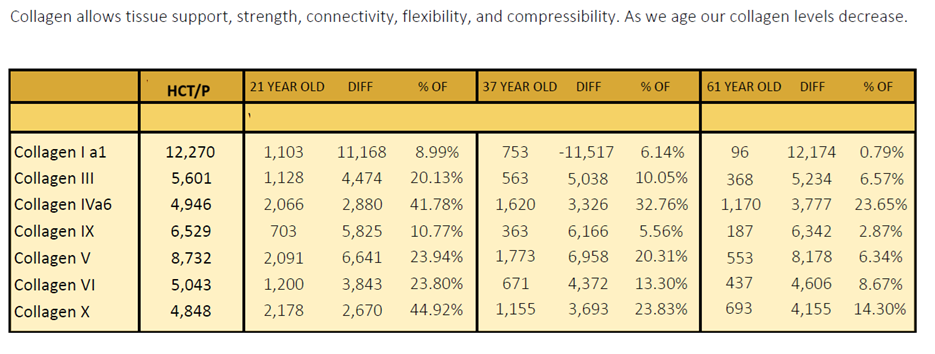
Quality Assurance:
These tissue products are processed from bio-ethically donated human birth tissue from healthy, consenting mothers in the United States who have passed a comprehensive medical background check, blood screenings and had full term, live C-Section births.
These products are processed in accordance with standards established by FDA and American Association of Tissue Banks (AATB) to minimize potential risks of disease transmission to recipients.
Infectious disease testing is performed at a certified laboratory in accordance with the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and 42 CFR part 493+. The products only come from a lab that is AATB accredited and Current Good Manufacturing Practices (cGMP) certified.
Benefits of HCT/P injections
- Treat many chronic pain disorders (knee/shoulder/hip/joint pain, back pain, neuropathy, etc)
- Promotes regenerative healing
- Decreases chronic inflammation
- Lubricates the joints
- Very safe with minimal to no side effects
- More growth factors then PRP injections
Call us today for consultation.